Search results
Search for "solid-phase synthesis" in Full Text gives 92 result(s) in Beilstein Journal of Organic Chemistry.
Synthesis and characterization of water-soluble C60–peptide conjugates
Beilstein J. Org. Chem. 2024, 20, 777–786, doi:10.3762/bjoc.20.71

- of hydrophilic oligopeptide anchors (oligo-Lys, oligo-Glu, and oligo-Arg) were synthesized. A previously reported Prato reaction adduct of a biscarboxylic acid-substituted C60 derivative was subjected to a solid phase synthesis for amide formation with N-terminal amines of peptides on resin to
Linker, loading, and reaction scale influence automated glycan assembly
Beilstein J. Org. Chem. 2023, 19, 1015–1020, doi:10.3762/bjoc.19.77
- purification steps. Keywords: automated glycan assembly; photocleavable linker; polysaccharides; solid-phase synthesis; Introduction Automated glycan assembly (AGA) is a solid-phase method that enables the rapid synthesis of complex oligo- and polysaccharides from protected monosaccharide building blocks
Synthesis of tetrahydrofuro[3,2-c]pyridines via Pictet–Spengler reaction
Beilstein J. Org. Chem. 2023, 19, 991–997, doi:10.3762/bjoc.19.74
- -c]pyridines. Moreover, multistep cascade processes with the simultaneous construction of several cores were described, where the key step is the generation of an acyliminium cation and the Pictet–Spengler cyclization [30][31][32], including solid-phase synthesis [33][34][35]. Another route for the
Solid-phase total synthesis and structural confirmation of antimicrobial longicatenamide A
Beilstein J. Org. Chem. 2022, 18, 1560–1566, doi:10.3762/bjoc.18.166
- longicatenamide A was confirmed. The developed solid-phase synthesis is expected to facilitate the rapid synthesis of diverse synthetic analogues. Keywords: antimicrobial; longicatenamides; peptidic natural product; solid-phase synthesis; total synthesis; Introduction Naturally occurring bioactive compounds can
- this study. Second, to realize the solid-phase synthesis, the C-terminus of the linear peptide was connected to 2-chlorotrityl resin [15] to give resin-bound peptide 5. Peptide 5 was divided into six building blocks 6–11 by retrosynthesis. At the beginning of the total synthesis, commercially
First series of N-alkylamino peptoid homooligomers: solution phase synthesis and conformational investigation
Beilstein J. Org. Chem. 2022, 18, 845–854, doi:10.3762/bjoc.18.85

- evaluated few segment-based coupling methods. The optimizations made with respect to the standard submonomer synthetic conditions will be useful for developing solid-phase synthesis and access longer and more diverse N-(alkylamino)peptoid oligomers in the future. NMR analysis of the synthesized oligomers 1
Synthesis and bioactivity of pyrrole-conjugated phosphopeptides
Beilstein J. Org. Chem. 2022, 18, 159–166, doi:10.3762/bjoc.18.17
- shown nucleobases, as the heteroaromatic groups, are able to act as the N-capping group for EISA of phosphopeptides [67]. In this study, we chose to examine a different type of heteroaromatic group, pyrroles, because pyrrole is adaptable for solid-phase synthesis [68] so that it is feasible to conjugate
- the designed peptides combines solution synthesis of the enzyme trigger or the fluorophore with the solid-phase synthesis of the pyrrole-peptide conjugates. We used phosphorus pentoxide and phosphoric acid to react with ᴅ-tyrosine, ʟ-tyrosine, ʟ-serine, or ᴅ-serine to produce ᴅ-phosphotyrosine, ʟ
- -phosphotyrosine, ʟ-phosphoserine, or ᴅ-phosphoserine, respectively. After being protected by an Fmoc group, these phosphorylated amino acids are suitable for solid-phase synthesis. We also used solution-phase synthesis to synthesize NBD-β-alanine according to the reported procedures [66]. We used standard Fmoc
First total synthesis of hoshinoamide A
Beilstein J. Org. Chem. 2021, 17, 2924–2931, doi:10.3762/bjoc.17.201
- synthesized in high efficiency. After systematic screening of the coupling reagents in liquid phase, the key intermediate tripeptide 7 was obtained in high yield. The solid-phase synthesis improves the entire efficiency of the synthetic route. This strategy could be applied to the stereoselective synthesis of
- . Solid-phase synthesis of hoshinoamide A from tripeptide 8 Tripeptide 8 (1.20 g, 2 mmol) was dissolved in a mixture of DCM (10 mL) and DMF (10 mL). DIPEA (1.7 mL, 10 mmol) and 2-CTC resin (1 g) were added to this mixture and the reaction was stirred at room temperature for 3 h. The resin was filtered and
Progress and challenges in the synthesis of sequence controlled polysaccharides
Beilstein J. Org. Chem. 2021, 17, 1981–2025, doi:10.3762/bjoc.17.129

- approach was efficient in solid phase as well as in solution phase synthesis. The participating (S)-(phenylthiomethyl) benzyl chiral auxiliary at the C-2 position of the glucosyl donor permitted the solid phase synthesis of a branched pentaglucan having a α(1–3) branch on an α(1–6) backbone [174]. Boron
Double-headed nucleosides: Synthesis and applications
Beilstein J. Org. Chem. 2021, 17, 1392–1439, doi:10.3762/bjoc.17.98
- phosphoramidites 160 and 164 which were then incorporated into oligodeoxynucleotides using automated solid phase synthesis. The synthesized oligonucleotides were removed from the solid support by treatment with concentrated aqueous ammonia which resulted in the formation of incorporated monomers 161 and 165 by
Beyond ribose and phosphate: Selected nucleic acid modifications for structure–function investigations and therapeutic applications
Beilstein J. Org. Chem. 2021, 17, 908–931, doi:10.3762/bjoc.17.76

- diseases. Oligonucleotides with alterations to their scaffold, prepared with modified nucleosides and solid-phase synthesis, have yielded molecules with interesting biophysical properties that bind to their targets and are tolerated by the cellular machinery to elicit a therapeutic outcome. Structural
- modifications N3' → P5' phosphoramidate The N3' → P5' phosphoramidate DNA (3'-NP DNA) contains a negatively charged internucleotide linkage, but one of the bridging oxygens is replaced by a nitrogen (Figure 1A). The 3'-NP linkage is generated during solid-phase synthesis where the incoming protected 5'-DMT-3
- synthesize the nucleoside dimer phosphoramidite with the appropriate amide linkage, which can then be introduced into the strand by solid-phase synthesis. These dimers are synthesized by using an amide coupling reagent to condense a 3'-carboxylic acid nucleoside with a 5'-amine nucleoside, where the
Synthesis and physicochemical evaluation of fluorinated lipopeptide precursors of ligands for microbubble targeting
Beilstein J. Org. Chem. 2021, 17, 511–518, doi:10.3762/bjoc.17.45

- of a perfluorohexane-stabilized microbubble with a fluorinated lipopeptide anchored in its phospholipid shell and b) structures of the perfluoroalkylated lipopeptides 1–3 and of the hydrocarbon analog 4. Solid-phase synthesis of F-lipopeptides 1–3 and hydrocarbon counterpart 4. Supporting
Synthetic strategies of phosphonodepsipeptides
Beilstein J. Org. Chem. 2021, 17, 461–484, doi:10.3762/bjoc.17.41
- phosphonodepsitripeptide with BOP as coupling reagent. Synthesis of norleucine-derived phosphonodepsipeptides 135 and 138. Synthesis of norleucine-derived phosphonodepsipeptides 141 and 144. Solid-phase synthesis of phosphonodepsipeptides. Synthesis of phosphonodepsidipeptides via the Mitsunobu reaction. Synthesis of γ
Incorporation of a metal-mediated base pair into an ATP aptamer – using silver(I) ions to modulate aptamer function
Beilstein J. Org. Chem. 2020, 16, 2870–2879, doi:10.3762/bjoc.16.236

- quadruplex, so that metal-mediated base pairs can be introduced. 3) It is a short oligonucleotide, so the modified aptamers can easily be synthesized using automated solid-phase synthesis. 4) The target molecule of this aptamer is a small molecule, facilitating the binding assays. 5) The aptamer also binds
- phosphoramidite-protected imidazole nucleoside as required for automated DNA solid-phase synthesis was synthesized as previously reported [30]. The oligonucleotides were synthesized on a DNA/RNA synthesizer H-8 (K&A Laborgeräte) using standard protocols for automated solid-phase synthesis (coupling time: 1000 s
Nonenzymatic synthesis of anomerically pure, mannosyl-based molecular probes for scramblase identification studies
Beilstein J. Org. Chem. 2020, 16, 1732–1739, doi:10.3762/bjoc.16.145
- into stereodefined, anomeric phosphoramidite derivatives. The method is based on adapted procedures developed for DNA solid-phase synthesis [17]. Phosphoramidite chemistry allows the connection of two molecular entities via a phosphodiester linkage and was found to be perfectly suited for this purpose
Photocontrolled DNA minor groove interactions of imidazole/pyrrole polyamides
Beilstein J. Org. Chem. 2020, 16, 60–70, doi:10.3762/bjoc.16.8
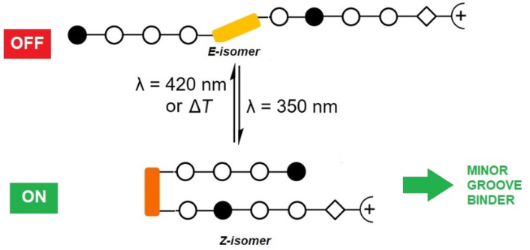
- -protected photoswitchable ω-amino acid 1 (Scheme 2A) was performed based on a procedure by Aemissegger et al. [35]. The ω-amino acid 1 was subsequently used in the solid-phase synthesis or for the synthesis of the dimer 7 in solution (Scheme 2B). The incorporation of β-alanine as a 'molecular spring' was
In search of visible-light photoresponsive peptide nucleic acids (PNAs) for reversible control of DNA hybridization
Beilstein J. Org. Chem. 2019, 15, 2500–2508, doi:10.3762/bjoc.15.243

- improved mismatch discrimination under physiological conditions than natural ones. Furthermore, PNAs have a straightforward chemical synthesis by Fmoc-based PNA solid-phase synthesis and remarkable stability against nuclease- and protease-mediated degradation [32][33]. In regard to all these beneficial
- . Black lines controls: solid: FAM-ssDNA without PNA and under the same conditions; dashed: BHQ/FAM-dsDNA. Solid-phase synthesis of photoswitchable PNAs; Aeg = N-(2-aminoethyl)glycine, Bhoc = benzhydryloxycarbonyl. Isomerization conversions at the photostationary state (PSS). Melting temperatures (TM) of
Synthesis and conformational preferences of short analogues of antifreeze glycopeptides (AFGP)
Beilstein J. Org. Chem. 2019, 15, 1581–1591, doi:10.3762/bjoc.15.162

- ., in food industry and biomedicine. Keywords: antifreeze glycopeptides; conformational preferences; NMR; PP II; solid phase synthesis; Introduction Certain species of polar and sub-polar fish created evolutionary strategies to survive in water at temperatures below the colligative freezing point
Electrophilic oligodeoxynucleotide synthesis using dM-Dmoc for amino protection
Beilstein J. Org. Chem. 2019, 15, 1116–1128, doi:10.3762/bjoc.15.108
- Shahien Shahsavari Dhananjani N. A. M. Eriyagama Bhaskar Halami Vagarshak Begoyan Marina Tanasova Jinsen Chen Shiyue Fang Department of Chemistry, Michigan Technological University, 1400 Townsend Drive, Houghton, Michigan 49931, USA 10.3762/bjoc.15.108 Abstract Solid-phase synthesis of
- thioester. Using the technology, the sensitive groups can be installed at any location within the ODN sequences without using any sequence- or functionality-specific conditions and procedures. Keywords: Dmoc; electrophilic; oligonucleotides; protecting group; solid-phase synthesis; Introduction After over
- sequences were very close and in some cases even overlapped, which made HPLC purification of the products difficult. A typical RP HPLC profile of ODNs synthesized in that manner is given in Supporting Information File 2. We therefore tried to keep the 5'-DMTr group at the end of solid phase synthesis to
Solid-phase synthesis of biaryl bicyclic peptides containing a 3-aryltyrosine or a 4-arylphenylalanine moiety
Beilstein J. Org. Chem. 2019, 15, 761–768, doi:10.3762/bjoc.15.72
- Iteng Ng-Choi Angel Oliveras Lidia Feliu Marta Planas LIPPSO, Departament de Química, University of Girona, Maria Aurèlia Capmany 69, Girona 17003, Spain 10.3762/bjoc.15.72 Abstract A methodology for the solid-phase synthesis of biaryl bicyclic peptides containing a Phe-Phe, a Phe-Tyr or a Tyr
- resulting biaryl monocyclic peptidyl resin leading to the formation of the expected biaryl bicyclic peptide. This study provides the first solid-phase synthesis of this type of bicyclic compounds being amenable to prepare a diversity of synthetic or natural biaryl bicyclic peptides. Keywords: borylation
- ; cross-coupling; cyclization; macrocycles; solid-phase synthesis; Introduction Monocyclic and bicyclic peptides are acquiring a relevant interest in current drug discovery. They display improved biological properties over their acyclic counterparts and, at the same time, they are suitable to modulate
Tuning the stability of alkoxyisopropyl protection groups
Beilstein J. Org. Chem. 2019, 15, 746–751, doi:10.3762/bjoc.15.70
- group of a nucleoside [7]. The acid lability of MIP is comparable to that of the dimethoxytrityl group (DMTr) that is frequently used to protect this position. The DMTr group works more or less perfectly in solid-phase synthesis, however, in solution-phase processes there are several cases where
Unexpected loss of stereoselectivity in glycosylation reactions during the synthesis of chondroitin sulfate oligosaccharides
Beilstein J. Org. Chem. 2019, 15, 137–144, doi:10.3762/bjoc.15.14
- . Therefore, alternative methodologies are still demanded in order to facilitate and accelerate intermediate purifications [23][24][25]. In this context, Seeberger and co-workers developed an automated solid-phase synthesis of CS derivatives using a photolabile linker [26]. Here, we describe the exploration
Ring-closing-metathesis-based synthesis of annellated coumarins from 8-allylcoumarins
Beilstein J. Org. Chem. 2018, 14, 2991–2998, doi:10.3762/bjoc.14.278
- these established methods necessary. Examples from the past 15 years include transition metal-catalyzed transformations [21][22][23], solid-phase synthesis directed at combinatorial library design [24] and organocatalytic annellation reactions [25][26]. Sparked by our interest in the development and
Novel solid-phase strategy for the synthesis of ligand-targeted fluorescent-labelled chelating peptide conjugates as a theranostic tool for cancer
Beilstein J. Org. Chem. 2018, 14, 2665–2679, doi:10.3762/bjoc.14.244

- ; found, 511.2640. General procedure for solid-phase synthesis Resin swelling All the resins used in solid-phase peptide synthesis were swelled initially with 5 mL of DCM for 30 minutes by bubbling nitrogen and after draining DCM, the resin is swelled once again with 5 mL DMF thrice for 15 minutes each
Phosphoramidite building blocks with protected nitroxides for the synthesis of spin-labeled DNA and RNA
Beilstein J. Org. Chem. 2018, 14, 1563–1569, doi:10.3762/bjoc.14.133
- are seen with DNA analogs 22c and 24c, in full accord with NUPACK predictions and the experimentally observed levels of modulation depth. Conclusion All three phosphoramidite building blocks 5, 7, and 8 are applicable for automated solid-phase synthesis using standard reaction cycles. As in the case
An overview of recent advances in duplex DNA recognition by small molecules
Beilstein J. Org. Chem. 2018, 14, 1051–1086, doi:10.3762/bjoc.14.93

- phase synthetic approach for the syntheses of cyclic Py/Im polyamides [84]. This group previously optimized and reported a machine-assisted Fmoc solid phase synthesis of simpler polyamides to afford high step-wise coupling yield [85]. A seven-member library of cyclic polyamides targeting androgen


























































































































































































































































































































































